Free Demo
Try PAS-X Savvy for one month
Our solution redefines the landscape of pharmaceutical process development & production by offering an all-in-one approach.
-
Efficient data management
-
User-friendly data visualization
-
Holistic data analysis
Unleash the Power of Seamless Data Management
Say goodbye to data management challenges with Werum PAS-X Savvy. Fast, comprehensive, and user-friendly, it's your tool for efficient bioprocess data handling and analytics.
PAS-X Savvy revolutionizes pharmaceutical process development, tech transfer, process validation, and production by streamlining processes holistically. It enhances communication, enables real-time bioprocess data management and analytics, ensuring an agile, secure transition from development to commercial production. This all-in-one solution harnesses the potential of process data, ensuring data integrity.




« Because of their complexity, we have to analyze data on a large number of parameters and from different sources, online and offline. Nevertheless, monitoring should happen automatically and holistically. With the help of PAS-X Savvy, we can monitor production parameters much more efficiently. »
Bryce Causey, Fermentation and Process Development Manager, New England Biolabs

Understanding the Core Modules
Explore the fundamental elements of PAS-X Savvy as we delve into its six key modules. These modules are GxP and Part 11 compliant and are crafted to enhance your pharmaceutical production experience, providing practical functionalities for data management, visualization, CPV trending, analysis, process modelling / digital twins and reporting. Join us in discovering the essential features that underlie PAS-X Savvy's straightforward approach to optimizing process development & production processes.
Manage
Data collection and contextualization of heterogeneous biopharmaceutical process data.
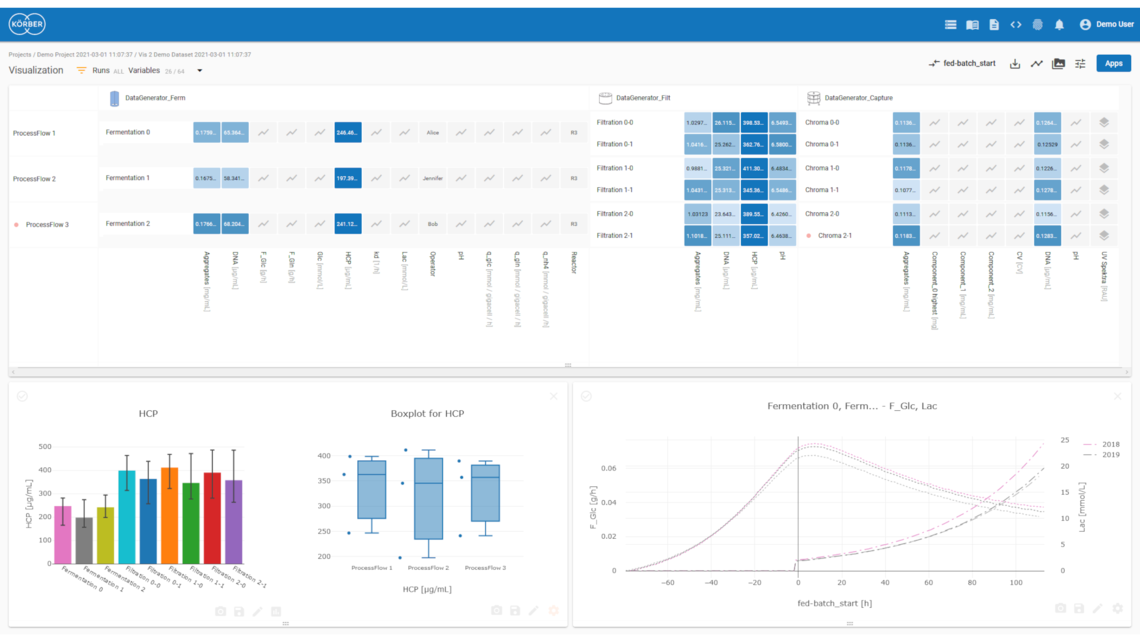
Visualize
Interactive, intuitive, and holistic visualization and dashboarding of time-series, sensor, quality, spectral, video, and image data for process development and manufacturing.
CPV
One application for monitoring and trending of Critical Quality Attributes (CQA) and other parameters for all unit operations, ensuring effective tracking of CPV trends.
Analyze
Data analytics for design of experiments (DoE), AI and mechanistic process modeling, MVDA, PCA, Golden Batch Comparison, Event & Phase Analysis, etc.
Report
Automatic and repetitive generation of reports for process development, regulatory filing, and product approval (PQR / APQR).
Integrated Process Modeling
End-to-end process modeling for accelerated process characterization (PCS) and adaptive, real-time control – from development to commercial manufacturing.
Join the pharmaceutical revolution with PAS-X Savvy. Optimize your processes, elevate your efficiency, and stay ahead in the dynamic landscape of pharmaceutical production.
Transform with confidence.
Frequently Asked Questions
Why do I need to fill out the information requested?
We will always keep your personal information safe.
We ask for your information in exchange for a valuable resource in order to
(a) improve your browsing experience by personalizing the Koerber site to your needs;
(b) send information to you that we think may be of interest to you by email or other means;
(c) send you marketing communications that we think may be of value to you. You can read more about our privacy policy here.
Is this really free?
Absolutely!
Just sharing some free knowledge that we hope you’ll find useful. Keep us in mind next time you have pharma manufacturing questions!
Who is this for?
This content offer is for anybody in pharma and biotech who is looking to reduce complexity of their bioprocess data management.
